Manganese oxides were used since antiquity as glaze ceramic brown/black pigments. In most of the cases, manganese oxide dissolves into the glaze but it is not uncommon to found manganese crystalline compounds associated to the dark decorations in historical ceramics. Kentrolite (Pb2Mn2Si2O9), hausmannite (Mn3O4) and braunite (Mn7SiO12) crystals are found in Islamic tin-lead-glazed pottery [1], [2]. Kentrolite and hausmannite are found in Hispano moresque pottery [3], in the oldest Swiss tin-opacified stove tiles [4] and in 16th century Portuguese productions [5]. Bustamite ((Mn,Ca)3Si3O9) is found in medieval 10th century production in la Vega de Granada [6], 14–15th century Portugal [7] and 13–14th century Barcelona productions [1], [2]. Braunite only is found in 17th century tin-lead glazes from Portugal, Barcelona and Hungary [1], [3], [8], [9] among others.
The nature, size and distribution of the manganese crystals depends on the composition of the pigment, glaze and ceramic substrate and firing protocol. MnO reacts with PbO and SiO2 and may produce manganese oxides (bixbyite, haussmanite), silicates (braunite) and lead silicates (kentrolite). However, CaO, MgO and Al2O3 are also commonly present in the high-lead glazes, either in the glass initial components or due to the reaction of the melt with the ceramic. They are commonly present as calcite, dolomite and clay or feldspar among others. Their incorporation increases the stability of the glaze but, it also gives rise to the precipitation of crystalline phases such as calcium and magnesium silicates and aluminosilicates (wollastonite, CaSiO3, diopside, CaMgSi2O6 and lead feldspar, PbAl2Si2O8), some of which are known to incorporate manganese in the structure (wollastonite, bustamite, diopside).
As kentrolite tend to dissolve at ∼950 °C [10] some attempts have been made based on the equilibrium phase diagrams available to correlate their presence with the firing temperature followed in historical glazed wares [1], [8], [11]. Even though the PbO-SiO2-MnO phase diagram does not exist, some other phase diagrams, such as PbO-SiO2 [12], [13], [14], [15], PbO-SiO2-CaO [16], [17], [18], PbO-SiO2-Al2O3 [19], SiO2-MnO [20], [21] and CaO-MnO-SiO2 [21], [22] do and can give only partial information about the compounds that may form.
Furthermore, high lead glazes were obtained either using lead compounds and quartz or a lead-glass. In the former case, many phases are produced during the heating, before the glaze melt and, in both cases, some phases may also be obtained during the cooling. Which manganese compounds are formed in each case and, whether they are produced during the heating or during the cooling is not known. In fact, kentrolite crystallites with different shapes have been found in some historical glazes. Some, growing around the manganese oxide particles with prismatic shape and some with a needle like shape [4], [8]. The later have been related to a possible fast-cooling process. Braunite crystallites also show two different morphologies in 17th century Catalan brown decorated ware: dark brown bipyramidal crystallites and brown thin lath-like crystallites [8].
The object of this study is to investigate the manganese compounds formed during the firing of a high-lead glaze and their possible role as a fingerprint of the materials and firing conditions used in the production of manganese decorated lead glazes. For this, a High Temperature Powder X-Ray Diffraction (HT-PXRD) experiment with Synchrotron Radiation has been designed to determine the phases formed and their stability range during the heating and cooling. Several mixtures of manganese oxide with lead oxide and quartz with a near eutectic high-lead glass composition to which calcite, dolomite and kaolinite were added, are studied. A mixture of manganese oxide with a 70PbO:30SiO2 glass is also studied.
The same mixtures were fired between 690 °C and 1020 °C following the same thermal path in a kiln at the laboratory. The resulting glazes were analysed by Scanning Electron Microscopy (SEM).
https://doi.org/10.1016/j.jeurceramsoc.2022.03.028
Abstract
A High Temperature Synchrotron Radiation X-Ray Powder Diffraction experiment was performed to determine the manganese compounds formed during the heating and cooling of 70 wt% PbO – 30 wt% SiO2 mixture or the equivalent glass plus 10 wt% of MnO. The effect of adding calcite, dolomite and kaolinite were also studied. All mixtures were fired between 690 °C and 1020 °C in oxidizing conditions and analysed by Scanning Electron Microscopy.
A sequence of manganese phases are formed during firing: bixbyite (Mn2O3), barysilite ((Pb,Mn)Si2O7), kentrolite (Pb2Mn2Si2O9) and braunite (Mn7SiO12). Kentrolite and braunite crystallise with different crystal habits during the heating and the cooling. If dolomite is present diopside ((Ca,Mg,Mn)2Si2O6) is formed. If calcite is present, ganomalite (Pb3(CaMn)2Si3O11), margarosanite (Pb(Ca,Mn)2Si3O9) and wollastonite ((Ca,Mn)SiO3) are also formed. Wollastonite can incorporate enough manganese to transform into bustamite ((Mn,Ca)3Si3O9) at high temperatures. This leaves less manganese available for the crystallisation of kentrolite and braunite.
Conclusions
Manganese oxide is a common compound used to colour and decorate historical glazed wares. Many different manganese phases have been identified in traditional high-lead glazes and their presence must be related to the materials used and firing protocol. The object of the study was to investigate their possible role as a fingerprint of the materials and firing conditions used in the production of manganese decorated lead glazes. For this, a High Temperature Synchrotron Powder X-Ray Diffraction experiment (HT-PXRD) was designed to determine the manganese compounds formed during the heating and the cooling of a mixture of manganese oxide with a near eutectic high-lead glass composition; calcite, dolomite and kaolinite were also added to study their role in the manganese compounds formed. To expand the firing temperature range above the range accessible in the HT-PXRD experiment (928 °C) the same mixtures were also fired in the laboratory following the same thermal protocol at temperatures (690–1020 °C) and were analysed by Scanning Electron Microscopy (SEM).
The manganese phases which will be found in a high-lead glaze are kentrolite (Pb2Mn2Si2O9) (above 700 °C) and braunite (Mn7SiO12) (above 900 °C). Both are formed by the reaction of manganese oxide with the lead-silicate melt, and consequently, either whether a lead oxide and quartz mixture or a lead-silicate glass is used. Kentrolite melts above 900 °C and, if fired below 980 °C, recrystallises during the cooling with a different crystal habit (feather like instead of prismatic). Above 980 °C only braunite, which grows during the cooling (dendritic structure), is found. In the calcitic mixture the sequence ganomalite (Pb3(CaMn)2Si3O11), margarosanite (Pb(Ca,Mn)2Si3O9) and wollastonite (above 850 °C) is observed while in the dolomitic mixture only diopsides are present at all temperatures. All these phases incorporate increasing amounts of manganese at higher temperatures, and at 1020 °C wollastonite incorporates enough manganese to transform into bustamite ((Mn,Ca)3Si3O9). The presence of manganese in those phases leaves less manganese available for the crystallisation of kentrolite and braunite. Finally, barysilite ((Pb,Mn)Si2O7) is only expected in lead glazes fired at low temperatures (below 850 °C). Relics of the manganese oxide particles can be found depending on their initial size appearing always as bixbyite (Mn2O3).
The presence and morphology of the manganese compounds found in high-lead glazes can be used to determine the firing conditions of traditional manganese decorations. The kinetic profile of the experiment is designed to provide the right sequence of manganese phases formed during heating and cooling, but not the exact temperature range which is heating rate dependent.
If the manganese content is low, once kentrolite is decomposed, there will not remain enough manganese in the melt to allow kentrolite recrystallisation and manganese will dissolve in the glaze completely. At higher temperatures, braunite will be found only if the melt contains enough manganese to produce some crystallites of braunite, otherwise, manganese will also dissolve in the glaze completely.
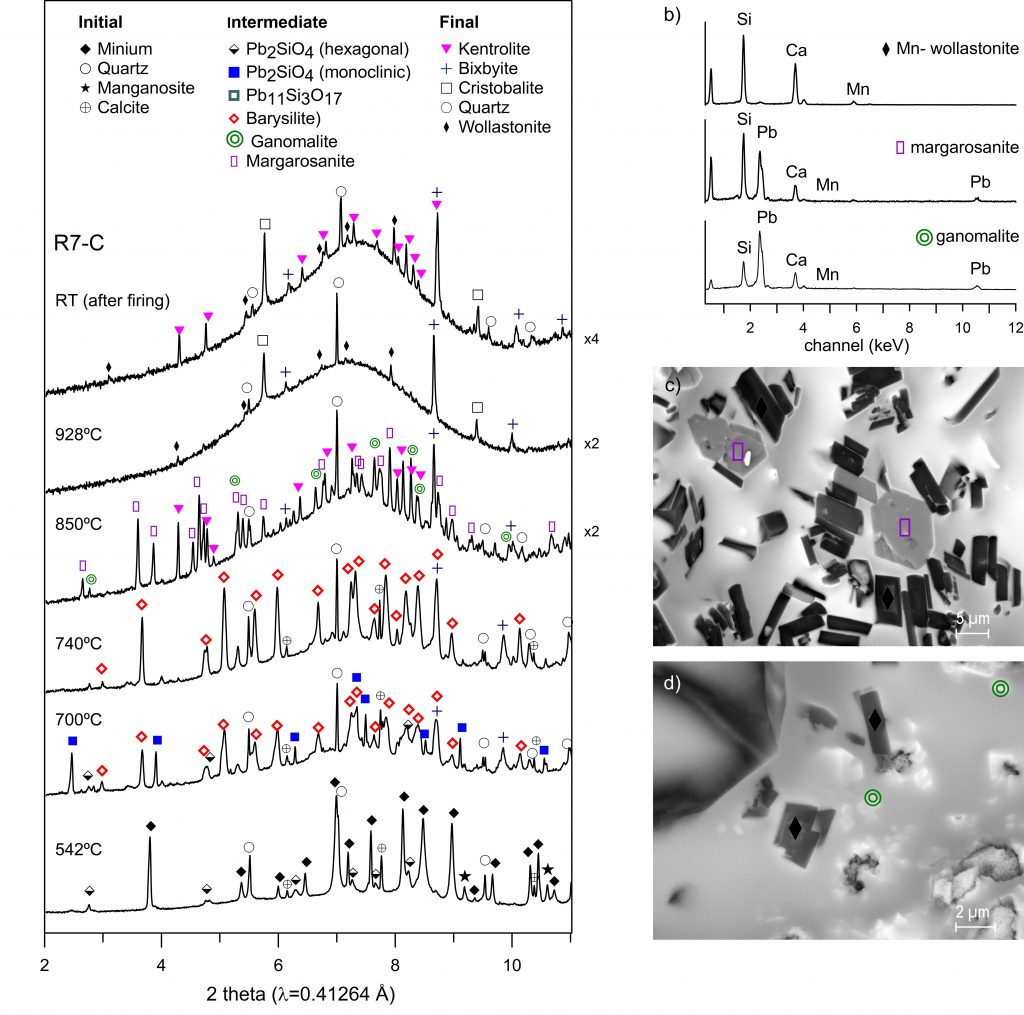
BSE images from the phases formed at (c) 928 ◦C (d) 850 ◦C and (e) 740 ◦C.
J. Molera et al.
Leave a Reply