The production of a lead glaze with galena: Thermal transformations in the PbS–SiO2 system
Roberta Di Febo, Judit Molera, Trinitat Pradell, Joan C. Melgarejo, Josep Madrenas, Oriol Vallcorba
First published: 4 December 2017Full publication history
DOI: 10.1111/jace.15346 View/save citation
Cited by (CrossRef): 0 articles Check for updates Citation tools
This article is part of the research results of the Doctoral Thesis of Roberta Di Febo
Abstract
Galena, also known as PbS, was widely used in the production of lead glazes from the beginning of the 18th century to the second half of the 20th century. Although the PbO-SiO2 system has been studied for years, the PbS–SiO2 phase diagram, involved in the formation of a glaze with galena, has not yet been investigated. Temperature transformations for the system 75 wt% PbS-25 wt% SiO2 are investigated in a high-temperature resolved X-ray diffraction experiment with synchrotron radiation and compared to those of the equivalent system 70 wt% PbO-30 wt% SiO2. Lanarkite, PbO·PbSO4, is the phase predominantly formed as soon as galena decomposes during the heating. The results show that the system melts at a temperature higher than the PbO–SiO2 system, but far lower than those expected for the PbO–PbSO4–PbS system. A historical misfired lead glaze produced with galena is also studied. The presence of galena, lanarkite, and mattheddleite, Pb10(SiO4)3.5(SO4)2Cl2, is determined and discussed in terms of the composition of the galena mineral used and the firing conditions in light of the high-temperature transformations previously obtained.
The production of a lead glaze with galena, PbS–SiO2 system, and the equivalent PbO–SiO2 system are investigated in a time-resolved high-temperature X-ray diffraction experiment with synchrotron radiation (HT-SR-XRD). Although the equilibrium phase diagram of the PbO–SiO2 system is well known, equilibrium conditions are not necessarily reached during the time-resolved experiment. Therefore, the data obtained for the PbO–SiO2 mixture will reveal any shift in the phase transformation and melting temperatures due to nonequilibrium conditions.
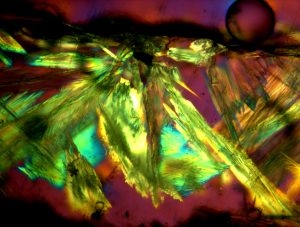
A historical misfired lead glaze ware that retains galena relic grains is also studied. The crystalline phases present in the glaze vestiges from an incomplete glaze firing are identified, and their presence is discussed in terms of the data obtained from the high-temperature experiment and of the composition of the glaze. Finally, the reasons for the use of galena in the production of lead glazes and its limitations are discussed in light of the results obtained.
Leave a Reply